Concept
Version 6
Created by Boundless
Dipole Moments
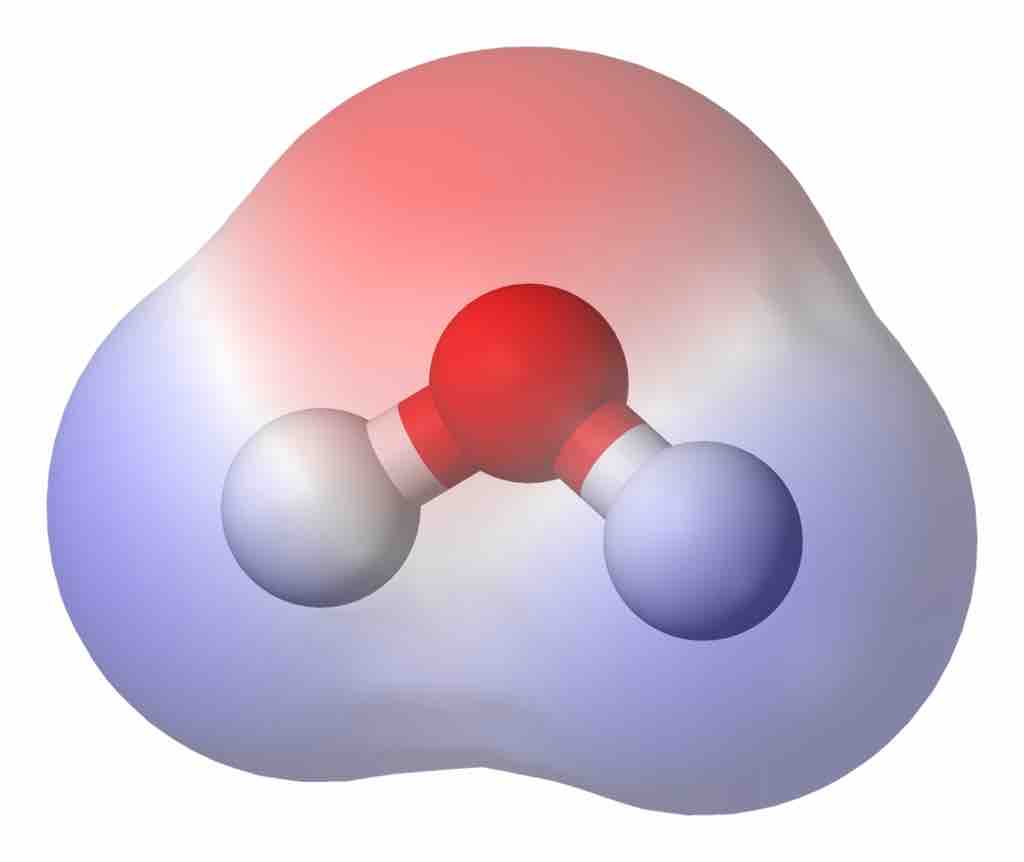
Molecular Dipole Moment in Water
This water (H2O) molecule has a high density of electrons (denoted by the red shading) near the red O atom. Closer to the white H atoms, there is a low density of electrons. Therefore, the molecule is is a dipole, with negativity near the O and positivity closer to the H atoms.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Water-elpot-transparent-3D-balls."
http://en.wikipedia.org/wiki/File:Water-elpot-transparent-3D-balls.png
Wikipedia
CC BY-SA.