Multielectron Atoms
Atoms with more than one electron, such as Helium (He) and Nitrogen (N), are referred to as multielectron atoms. Hydrogen is the only atom in the periodic table that has one electron in the orbitals under ground state.
In hydrogen-like atoms (those with only one electron), the net force on the electron is just as large as the electric attraction from the nucleus. However, when more electrons are involved, each electron (in the
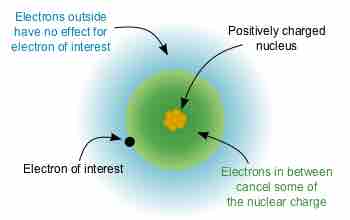
Electron Shielding Effect
A multielectron atom with inner electrons shielding outside electrons from the positively charged nucleus
The size of the shielding effect is difficult to calculate precisely due to effects from quantum mechanics. As an approximation, the effective nuclear charge on each electron can be estimated by: Zeff=Z−σZ_\text{eff} = Z - \sigma, where
For example, consider a sodium cation, a fluorine anion, and a neutral neon atom. Each has 10 electrons, and the number of nonvalence electrons is two (10 total electrons minus eight valence electrons), but the effective nuclear charge varies because each has a different number of protons:
As a consequence, the sodium cation has the largest effective nuclear charge and, therefore, the smallest atomic radius.