The electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals. Electron configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals.
In atoms, electrons fill atomic orbitals according to the Aufbau principle (shown in ), stated as: a maximum of two electrons are put into orbitals in the order of increasing orbital energy—the lowest-energy orbitals are filled before electrons are placed in higher-energy orbitals. As an example, the electron configuration of the neon atom is 1s2 2s2 2p6 or [He]2s2 2p6 , as diagramed in . In molecules, the situation becomes more complex, as each molecule has a different orbital structure. The molecular orbitals are labelled according to their symmetry, rather than the atomic orbital labels used for atoms and monoatomic ions: hence, the electron configuration of the diatomic oxygen molecule, O2, is 1σg2 1σu2 2σg2 2σu2 1πu4 3σg2 1πg2.
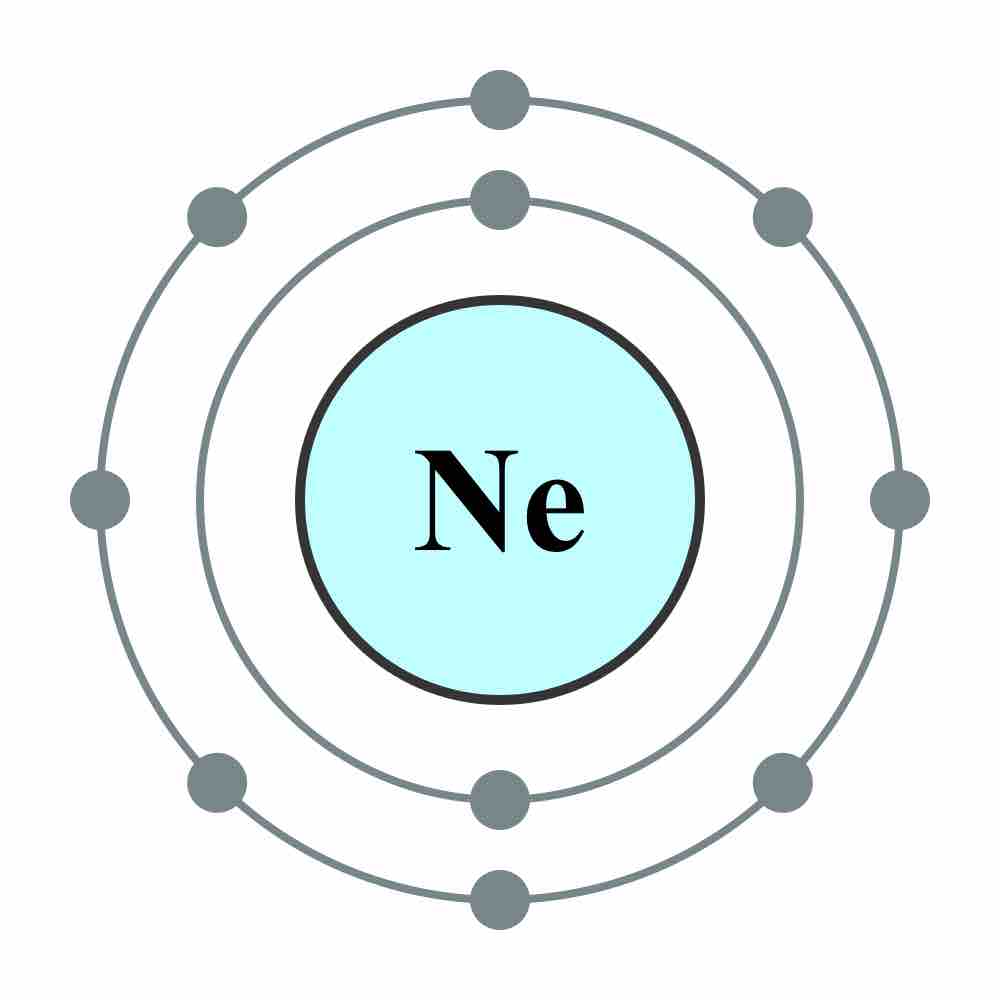
Electron Configuration of Neon Atom
Electron configuration of neon atom showing only outer electron shell.

Aufbau Principle
In the Aufbau Principle, as electrons are added to atoms, they are added to the lowest orbitals first.
According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by emission or absorption of a quantum of energy, in the form of a photon.
For atoms or molecules with more than one electron, the motion of electrons are correlated and such picture is no longer exact. An infinite number of electronic configurations are needed to exactly describe any multi-electron system, and no energy can be associated with one single configuration. However, the electronic wave function is usually dominated by a very small number of configurations and therefore the notion of electronic configuration remains essential for multi-electron systems.
Electronic configuration of polyatomic molecules can change without absorption or emission of photon through vibronic couplings.
Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The outermost electron shell is often referred to as the valence shell and (to a first approximation) determines the chemical properties. It should be remembered that the similarities in the chemical properties were remarked more than a century before the idea of electron configuration. The concept of electron configuration is also useful for describing the chemical bonds that hold atoms together. In bulk materials this same idea helps explain the peculiar properties of lasers and semiconductors.