Autoimmunity is the failure of an organism in recognizing its own constituent parts as self, creating an immune response against its own cells and tissues. Any disease that results from such an aberrant immune response is termed an autoimmune disease.
Autoimmunity is often caused by a lack of germ development of a target body and, as such, the immune response acts against its own cells and tissues. Prominent examples include Coeliac disease, diabetes mellitus type 1 (IDDM), Sarcoidosis, systemic lupus erythematosus (SLE), Sjögren's syndrome, Churg-Strauss Syndrome, Hashimoto's thyroiditis, Graves' disease, idiopathic thrombocytopenic purpura, Addison's Disease, rheumatoid arthritis (RA) and allergies.
Autoimmune diseases are very often treated with steroids. The misconception that an individual's immune system is totally incapable of recognizing self antigens is not new. Paul Ehrlich, at the beginning of the twentieth century, proposed the concept of horror autotoxicus, wherein a 'normal' body does not mount an immune response against its own tissues. Thus, any autoimmune response was perceived to be abnormal and postulated to be connected with human disease. Now, it is accepted that autoimmune responses are an integral part of vertebrate immune systems (sometimes termed 'natural autoimmunity'), normally prevented from causing disease by the phenomenon of immunological tolerance to self-antigens.
Autoimmunity should not be confused with alloimmunity. Certain individuals are genetically susceptible to developing autoimmune diseases. This susceptibility is associated with multiple genes plus other risk factors. Genetically predisposed individuals do not always develop autoimmune diseases. Three main sets of genes are suspected in many autoimmune diseases: immunoglobulins, T-cell receptors and the major histocompatibility complexes (MHC).
Immunoglobulins and the T-cell receptors are involved in the recognition of antigens and they are inherently variable and susceptible to recombination. These variations enable the immune system to respond to a very wide variety of invaders, but may also give rise to lymphocytes capable of self-reactivity. Scientists such as H. McDevitt, G. Nepom, J. Bell and J. Todd have also provided strong evidence to suggest that certain MHC class II allotypes are strongly correlated with autoimmunity.
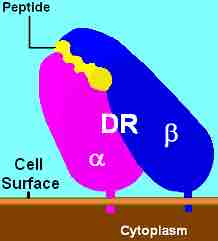
MHC Class II, DR
HLA-DR is a MHC class II cell surface receptor encoded by the human leukocyte antigen complex on chromosome 6 region 6p21.31. The complex of HLA-DR and its ligand, a peptide of 9 amino acids in length or longer, constitutes a ligand for the T-cell receptor (TCR).
For example:
1. HLA DR2 is strongly positively correlated with Systemic Lupus Erythematosus, narcolepsy and multiple sclerosis, and negatively correlated with DM Type 1.
2. HLA DR3 is correlated strongly with Sjögren's syndrome, myasthenia gravis, SLE, and DM Type 1.
3. HLA DR4 is correlated with the genesis of rheumatoid arthritis, Type 1 diabetes mellitus, and pemphigus vulgaris.
Fewer correlations exist with MHC class I molecules. The most notable and consistent is the association between HLA B27 and ankylosing spondylitis. Correlations may exist between polymorphisms within class II MHC promoters and autoimmune disease. The contributions of genes outside the MHC complex remain the subject of research both in animal models of disease (Linda Wicker's extensive genetic studies of diabetes in the NOD mouse), and in patients (Brian Kotzin's linkage analysis of susceptibility to SLE).
A person's sex also seems to have some role in the development of autoimmunity, classifying most autoimmune diseases as sex-related diseases. Nearly 75% of the more than 23.5 million Americans who suffer from autoimmune disease are women, although it is less-frequently acknowledged that millions of men also suffer from these diseases.
According to the American Autoimmune Related Diseases Association (AARDA), autoimmune diseases that develop in men tend to be more severe. A few autoimmune diseases that men are just as or more likely to develop as women, include: ankylosing spondylitis, type 1 diabetes mellitus, Wegener's granulomatosis, Crohn's disease, Primary sclerosing cholangitis and psoriasis.
The reasons for the sex role in autoimmunity are unclear. Women appear to generally mount larger inflammatory responses than men when their immune systems are triggered, increasing the risk of autoimmunity. Similarly, involvement of sex steroids is indicated by the fact that many autoimmune diseases tend to fluctuate in accordance with hormonal changes. Interestingly, a history of pregnancy also appears to leave a persistent increased risk for autoimmune disease. Indeed, it has been suggested that the slight exchange of cells between mothers and their children during pregnancy may induce autoimmunity. This would tip the gender balance in the direction of the female.
Another theory suggests the female-high tendency to get autoimmunity is due to an imbalanced X chromosome inactivation. The X-inactivation skew theory, proposed by Princeton University's Jeff Stewart, has recently been confirmed experimentally in scleroderma and autoimmune thyroiditis.