Section 2
Chemical Bonds
Book
Version 6
By Boundless
By Boundless
Boundless Microbiology
Microbiology
by Boundless
5 concepts
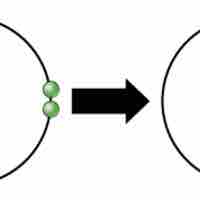
Ions and Ionic Bonds
Ionic bonds are attractions between oppositely charged atoms or groups of atoms where electrons are donated and accepted.
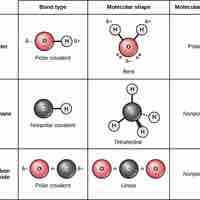
Covalent Bonds and Other Bonds and Interactions
Covalent bonds result from a sharing of electrons between two atoms and hold most biomolecules together.
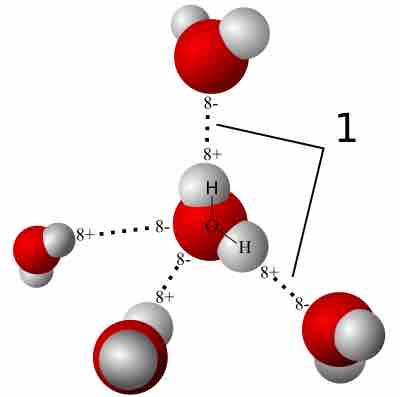
Hydrogen Bonding
A hydrogen bond is a strong intermolecular force created by the relative positivity of hydrogen atoms.

Avogadro's Number and the Mole
The mole is represented by Avogadro's number, which is 6.02×1023 mol-1.

Average Atomic Mass
The average atomic mass of an element is the sum of the masses of its isotopes, each multiplied by its natural abundance.