Reverse transcriptase is an enzyme that has the ability to transcribe single-stranded DNA from a single-stranded RNA chain. This is the reverse of the usual flow of information when RNA is synthesized from DNA. Viruses that use reverse transcriptase to convert their genetic material (RNA) into DNA are called retroviruses. One of the most prominent representative of a retrovirus is HIV. Due to the high prevalence of HIV/AIDS in the world, it is important to have drugs that will prevent or cure the infection . This enzyme is also found in tumors and cancer cells.
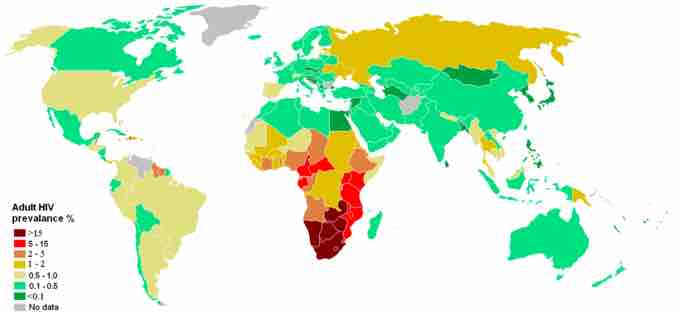
Estimation of adults with HIV/AIDS by country
The data shows the people, between 18-49 years, by country who are affected by HIV/AIDS. The information is provided by UNAIDS based on a report from July 2008
Drugs that inhibit the function of this enzyme are divided into three groups:
- nucleoside analog reverse transcriptase inhibitors
- nucleotide analog reverse transcriptase inhibitors
- non-nucleoside reverse transcriptase inhibitors
The first two inhibitors act on the same principle. They mimic, respectively, nucleosides or nucleotides but lack a free hydroxyl group at the 3' end. The major difference between them is that the nucleosides need to be phosphorylated by cellular kinases. The enzyme reverse transcriptase recognizes them as regular nucleotides and inserts them into the newly synthesized DNA chain. But once inserted the elongation stops at them because no more nucleotides can be added due to the lack of the 3' hydroxyl group and the inability of the formation of 5'-3' phosphodiester bond. This process is called chain termination. Nucleoside and nucleotide inhibitors are also called competitive substrate inhibitors. Examples of such drugs are Zidovudine (AZT) and Lamivudine. AZT was the first FDA approved drug for the treatment of HIV. Lamivudine is used for the treatment of both HIV and hepatitis B. Since some viruses, such as hepatitis B, carry RNA-dependent DNA polymerases reverse transcriptase inhibitors can be used to treat these infections as well.
Non-nucleotide reverse transcriptase inhibitors bind to a different site, not the active one, of the reverse transcriptase enzyme. That leads to conformational changes that distort the position of the DNA binding sites in the enzyme and lead to halt in DNA polymerization. Non-nucleotide inhibitors are non-competitive inhibitorsof reverse transcriptase. Such drugs are Efavirenz and Nevirapine.
Resistance occurs to all drug groups. The mechanisms for resistance against the nucleoside (nucleotide) inhibitors are two. The first one is due to mutations in the N-terminal polymerase domain of the reverse transcriptase that makes it less likely to incorporate the analogues. The second mechanism is caused by mutations in the transcriptase that allow the removal of the incorporated inhibitor and hence restart of DNA replication.
Resistance to the non-nucleotide inhibitors is caused by mutations in the inhibitor binding site of the enzyme. Such mutations prevent the binding of the inhibitor to the enzyme.