Transition Metal Properties
There are a number of properties shared by the transition elements that are not found in other elements, which result from the partially filled d subshell. These include the formation of compounds whose color is due to d–d electronic transitions and the formation of many paramagnetic compounds due to the presence of unpaired d electrons. Color in transition-series metal compounds is generally due to electronic transitions of two principal types: charge-transfer transitions and d-d transitions.

Colors of transition metal compounds
From left to right, aqueous solutions of: Co(NO3)2 (red); K2Cr2O7 (orange); K2CrO4 (yellow); NiCl2 (turquoise); CuSO4 (blue); KMnO4 (purple).
Charge Transfer Transitions
An electron may jump from a predominantly ligand orbital to a predominantly metal orbital, giving rise to a ligand-to-metal charge-transfer (LMCT) transition. These can most easily occur when the metal is in a high oxidation state. For example, the color of chromate, dichromate, and permanganate ions is due to LMCT transitions. In each case the metals (Cr and Mn) have oxidation states of +6 or higher.
A metal-to ligand charge transfer (MLCT) transition will be most likely when the metal is in a low oxidation state and the ligand is easily reduced.
d-d Transitions
In a d-d transition, an electron jumps from one d-orbital to another. In complexes of the transition metals, the d orbitals do not all have the same energy. The pattern of splitting of the d orbitals can be calculated using crystal field theory. The extent of the splitting depends on the particular metal, its oxidation state, and the nature of the ligands.
In centrosymmetric complexes, such as octahedral complexes, d-d transitions are forbidden. Tetrahedral complexes have a somewhat more intense color because mixing d and p orbitals is possible when there is no center of symmetry, so transitions are not pure d-d transitions.
Some d-d transitions are spin forbidden. An example occurs in octahedral, high-spin complexes of manganese(II) in which all five electrons have parallel spins. The color of such complexes is much weaker than in complexes with spin-allowed transitions. In fact, many compounds of manganese(II) appear almost colorless.
Transition metal compounds are paramagnetic when they have one or more unpaired d electrons. In octahedral complexes with between four and seven d electrons, both high spin and low spin states are possible. Tetrahedral transition metal complexes, such as [FeCl4]2−, are high-spin because the crystal field splitting is small. This means that the energy to be gained by virtue of the electrons being in lower energy orbitals is always less than the energy needed to pair up the spins.
Paramagnetic vs. Diamagnetic
Some compounds are diamagnetic. In these case all of the electrons are paired up. Ferromagnetism occurs when individual atoms are paramagnetic and the spin vectors are aligned parallel to each other in a crystalline material. Metallic iron is an example of a ferromagnetic material involving a transition metal. Anti-ferromagnetism is another example of a magnetic property arising from a particular alignment of individual spins in the solid state.
As implied by the name, all transition metals are metals and conductors of electricity. In general, transition metals possess a high density and high melting points and boiling points. These properties are due to metallic bonding by delocalized d electrons, leading to cohesion which increases with the number of shared electrons. However, the Group 12 metals have much lower melting and boiling points since their full d subshells prevent d–d bonding. In fact, mercury has a melting point of −38.83 °C (−37.89 °F) and is a liquid at room temperature.

Ferromagnetism
A magnet made of alnico, an iron alloy. Ferromagnetism is the physical theory which explains how materials become magnets.
Transition Metals and Atomic Size
In regards to atomic size of transition metals, there is little variation. Typically, when moving left to right across the periodic table, there is a trend of decreasing atomic radius. However, in the transition metals, moving left to right, there is a trend of increasing atomic radius which levels off and becomes constant. In the transition elements, the number of electrons are increasing but in a particular way. The number of electrons increase going across a period, thus, there is more pull of these electrons towards the nucleus. However, with the d−electrons, there is some added electron-electron repulsion. For example, in chromium, there is a promotion of one of the 4s electrons to half fill the 3d sublevel; the electron-electron repulsions are less and the atomic size is smaller. The opposite holds true for the latter part of the row.
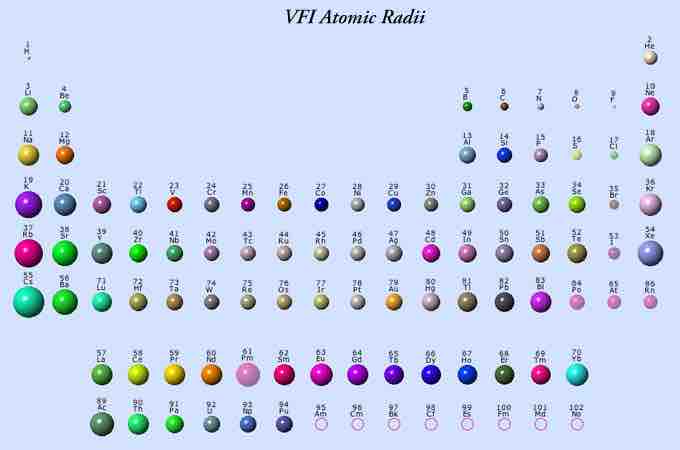
Periodic table of elements
This image represents atomic radii size. Note the size of the transition metals.