Magnetic Properties of Coordination Compounds
An interesting characteristic of transition metals is their ability to form magnets. Metal complexes that have unpaired electrons are magnetic. Since the last electrons reside in the d orbitals, this magnetism must be due to having unpaired d electrons. Considering only monometallic complexes, unpaired electrons arise because the complex has an odd number of electrons or because electron pairing is destabilized.
For example, monomeric Ti(III) species have one d electron and must be (para)magnetic, regardless of the geometry or the nature of the ligands. Ti(II), with two d electrons, forms some complexes that have two unpaired electrons and others with none.
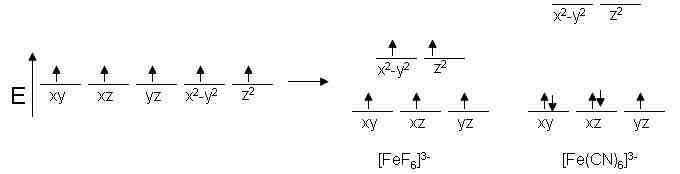
As an example, Fe prefers to exist as Fe3+ and is known to have a coordination number of six. Since the configuration of Fe3+ has five d electrons, we would expect to see five unpaired spins in complexes with Fe. This is true for [FeF6]3-; however, [Fe(CN)6]3- only has one unpaired electron, making it a weaker magnet. This trend can be explained based on the properties of the ligands. We expect CN− to have a stronger electric field than that of F−, so the energy differences in the d orbitals should be greater for the cyanide complex.
Crystal field theory splitting diagram
Example of influence of ligand electronic properties on d orbital splitting. This shows the comparison of low-spin versus high-spin electrons.
In order for this to make sense, there must be some sort of energy benefit to having paired spins for our cyanide complex. That is, the energy level difference must be more than the repulsive energy of pairing electrons together. Since systems strive to achieve the lowest energy possible, the electrons will pair up before they will move to the higher orbitals. This is referred to as low spin, and an electron moving up before pairing is known as high spin.
Tetrahedral complexes have naturally weaker splitting because none of the ligands lie within the plane of the orbitals. As a result, they have either have too many or too few d electrons to warrant worrying about high or low spin. Square planar compounds, on the other hand, stem solely from transition metals with eight d electrons. [Ni(CN)4]2-, [Pt(NH3)3Cl]+, and [PtCl4]2- are all diamagnetic.
Since this encompasses the full spectrum of ligand strength, we can conclude that square planar compounds are always low spin and therefore are weakly magnetic. In bi- and polymetallic complexes, in which the individual centers have an odd number of electrons or electrons are high-spin, the situation is more complicated.
If there is interaction between the two (or more) metal centers, the electrons may couple, resulting in a weak magnet, or they may enhance each other. When there is no interaction, the two (or more) individual metal centers behave as if in two separate molecules.