Concept
Version 13
Created by Boundless
Standard Free Energy Changes
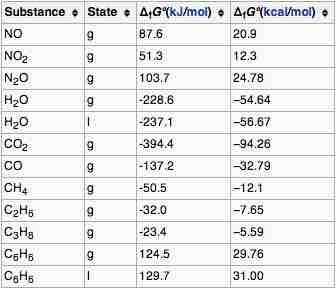
Gibbs Energy of Formation
The standard Gibbs free energy of formation of a compound is the change of Gibbs free energy that accompanies the formation of 1 mole of that substance from its component elements, at their standard states.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources: