The heat of solution, also referred to the enthalpy of solution or enthalpy of dissolution, is the enthalpy change associated with the dissolution of a solute in a solvent at constant pressure, resulting in infinite dilution. The heat of solution, like all enthalpy changes, is expressed in kJ/mol for a reaction taking place at standard conditions (298.15 K and 1 bar).
Three-Step Process of Dissolution
The heat of solution can be regarded as the sum of the enthalpy changes of three intermediate steps:
- The breaking of bonds within the solute, such as the electrostatic attraction between two ions (endothermic)
- The breaking of intermolecular attractive forces within the solvent, such as hydrogen bonds (endothermic)
- The formation of new attractive solute-solvent bonds in solution (exothermic)
The value of the overall heat of solution,
If more energy is released in making bonds than is used in breaking bonds, the overall process is exothermic, and ∆Hsol is negative. If more energy is used in breaking bonds than is released upon solute-solvent bond formation, then the overall process is endothermic, and ∆Hsol is positive.
Examples
- Dissolution of sodium chloride (table salt) in water is endothermic. This is because the amount of energy used to break apart the hydrogen bonding interactions between water molecules, as well as the energy used to break apart the electrostatic attractions between sodium and chloride ions, is greater than the amount of energy released when new solute-solvent attractions are formed between water molecules and aqueous ions in solution.
- Dissolving potassium hydroxide is exothermic. This is because more energy is released upon formation of solute-solvent bonds than was required to break apart the hydrogen bonds in water, as well as the ionic bonds in KOH.
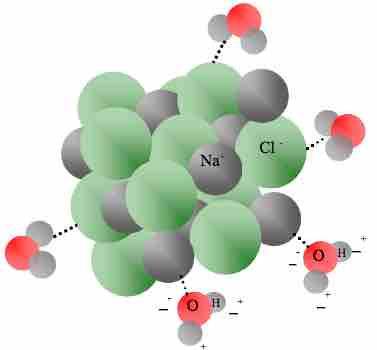
Dissolution of NaCl in water
Dissolution of sodium chloride in water is endothermic. Solute-solvent attractive bond formation (the exothermic step in the process of solvation) is indicated by dashed lines.