Concept
Version 9
Created by Boundless
Solutions and Heats of Hydration
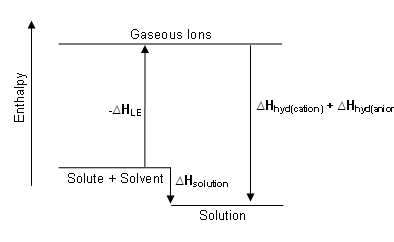
Enthalpy diagram for the dissolution process
The enthalpy diagram showing exothermic solution formation. Notice that Hsolution is lower in energy than the starting solute/solvent enthalpies. An endothermic process, on the other hand, would show Hsolution as positive, and it would be higher in energy than the starting solute/solvent enthalpies.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Lattice NRG for thermodynamic rxn."
http://www.4college.co.uk/a/O/energy.php
Salter
Free Art License.