Amino acids are the building blocks for the proteins responsible for the biological functions within our body. Amino acids are chemical compounds consisting of a carbon atom bonded to an amine group, a hydrogen atom, a carboxylic group, and a varying side-chain (R group); it is this side chain that distinguishes each amino acid from another. Higher-ordered structures such as peptide chains and proteins are formed when amino acids bond to each other.
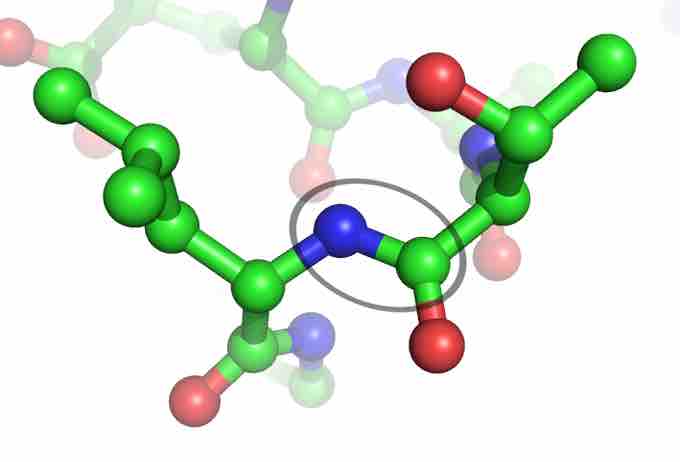
The Peptide Bond
The peptide bond (circled) links two amino acids together. The blue balls represent the nitrogen that connect from the amine terminus of one amino acid to the carboxylate of another. The green balls are carbon, and the red are oxygen.
Peptides
A peptides is a molecule composed of two or more amino acids. The bond that holds together the two amino acids is a peptide bond, or a covalent chemical bond between two compounds (in this case, two amino acids). It occurs when the carboxylic group of one molecule reacts with the amino group of the other molecule, linking the two molecules and releasing a water molecule.
Long chain polypeptides can be formed by linking many amino acids to each other via peptide bonds. The amide bond can only be broken by amide hydrolysis, where the bonds are cleaved with the addition of a water molecule. The peptide bonds of proteins are metastable, and will break spontaneously in a slow process. Living organisms have enzymes which are capable of both forming and breaking peptide bonds .
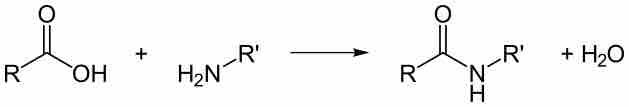
The Amide Bond
Peptide bonds are amide bonds, characterized by the presence of a carbonyl group attached to an amine.
Resonance Forms of the Amid Group
The amide group has three resonance forms, which confer important properties. First, the stabilization afforded from the resonance structures effectively stabilizes it by 80kj/mol, making it less reactive than similar groups. The peptide bond is uncharged at normal pH values, but the double bonded character from the resonance structure creates a dipole, which can line up in secondary structures. The partial double bond character can be strengthened or weakened by modifications that favor one another, allowing some flexibility for the presence of the peptide group in varying conditions. The extra stabilization makes the peptide bond relatively stable and unreactive. However, peptide bonds can undergo chemical reactions, typically through an attack of the electronegative atom on the carbonyl carbon, resulting in the formation of a tetrahedral intermediate.