The periodic table of elements has a total of 118 entries. Elements are arranged in a series of rows (periods) in order of atomic number so that those with similar properties appear in vertical columns. Elements in the same period have the same number of electron shells; moving across a period (so progressing from group to group), elements gain electrons and protons and become less metallic. This arrangement reflects the periodic recurrence of similar properties as the atomic number increases. For example, the alkali metals lie in one group (Group 1) and share similar properties, such as high reactivity and the tendency to lose one electron to arrive at a noble-gas electron configuration.
Modern quantum mechanics explains these periodic trends in properties in terms of electron shells. The filling of each shell corresponds to a row in the table.
In the s-block and p-block of the periodic table, elements within the same period generally do not exhibit trends and similarities in properties (vertical trends down groups are more significant). However, in the d-block, trends across periods become significant, and the f-block elements show a high degree of similarity across periods (particularly the lanthanides).
If we examine the physical state of each element, we notice that on the left side of the table, elements such as lithium and beryllium are metallic solids, whereas on the right, nitrogen, oxygen, fluorine, and neon are all gases. This is because lithium and beryllium form metallic solids, whereas the elements to the right form covalent compounds with little intermolecular force holding them together. Therefore we can say that, in general, elements tend to go from solids to liquids to gases as we move across a given period. However, this is not a strict trend.
Bonding
As you move across a period in the periodic table, the types of commonly encountered bonding interactions change. For example, at the beginning of Period 2, elements such as lithium and beryllium form only ionic bonds, in general. Moving across the period, elements such as boron, carbon, nitrogen and oxygen tend to form covalent bonds. Fluorine can form ionic bonds with some elements, such as carbon and boron, and neon does not tend to form any bonds at all.
Melting Points of the Halides
Another physical property that varies across a period is the melting point of the corresponding halide. A halide is a binary compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluoride, chloride, bromide, iodide, or astatide compound. Many salts are halides; the hal- syllable in halide and halite reflects this correlation. All Group 1 metals form halides that are white solids at room temperature.
The melting point is correlated to the strength of intermolecular bonds within the element. First, we must analyze compounds formed from elements from Groups 1 and 2 (e.g., sodium and magnesium). To develop an understanding of bonding in these compounds, we focus on the halides of these elements. The physical properties of the chlorides of elements in Groups 1 and 2 are very different compared to the chlorides of the elements in Groups 4, 5, and 6.
All of the alkali halides and alkaline earth halides are solids at room temperature and have melting points in the hundreds of degrees centigrade. For example, the melting point of sodium chloride (NaCl) is 808 °C. In contrast, the melting points of the non-metal halides from Periods 2 and 3, such as CCl4, PCl3, and SCl2, are below 0 °C, so these materials are liquids at room temperature. Furthermore, all of these compounds have low boiling points, typically in the range of 50 °C to 80 °C.
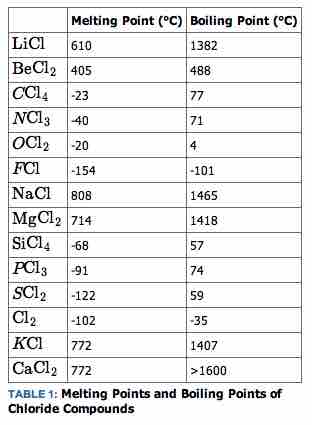
Melting and boiling points of various halides
The melting points of a series of chloride compounds.
The non-metal halide liquids are also electrical insulators and do not conduct electrical current. In contrast, when an alkali halide or alkaline earth halide melts, the resulting liquid is an excellent electrical conductor. This tells us that these molten compounds consist of ions, whereas the non-metal halides do not. This again demonstrates the type of bonding that these compounds exhibit: the left-most elements form more ionic bonds, and the further-right elements tend to form more covalent bonds.