Section 2
Molar Mass
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
4 concepts

Avogadro's Number and the Mole
The mole is represented by Avogadro's number, which is 6.02×1023 mol-1.
Converting between Moles and Atoms
By understanding the relationship between moles and Avogadro's number, scientists can convert between number of moles and number of atoms.
Molar Mass of Compounds
The molar mass of a particular substance is the mass of one mole of that substance.
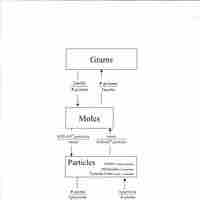
Converting between Mass and Number of Moles
A substance's molar mass can be used to convert between the mass of the substance and the number of moles in that substance.