Mass spectrometry (MS) is a powerful technique that can identify a wide variety of chemical compounds. It is used to determine a particle's mass, the elemental composition of a sample, and the chemical structures of larger molecules.
Mass spectrometers separate compounds based on a property known as the mass-to-charge ratio: the mass of the atom divided by its charge. First, the sample is ionized. Ionization is the process of converting an atom or molecule into an ion by adding or removing charged particles such as electrons or ions. Once the sample is ionized, it is passed through some form of electric or magnetic field. A particle's mass can be calculated based on parameters such as how long it takes to travel a certain distance or its angle of travel.
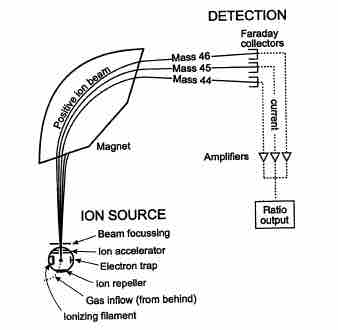
Schematic of Mass Spectrometer
A sample is loaded onto the mass spectrometer, where it undergoes vaporization and ionization. The components of the sample are ionized by one of a variety of methods, such as the ionizing filament. The ions are separated in an analyze by magnetic fields. They are separated according to their mass-to-charge ratios. The ions are detected, usually by a quantitative method such as a Faraday collector. The ion signal is processed into a mass spectrum.
The Make-Up of Mass Spectrometry (MS) Instruments
MS instruments consist of two main components:
- An ion source, which can convert sample molecules into ions
- A mass analyzer, which sorts the ions by mass by applying electromagnetic fields
There are a wide variety of techniques for ionizing and detecting compounds.
Ionizing Compounds
The ion source is the part of the mass spectrometer that ionizes the compound. Depending on the information desired from mass spectrometry analysis, different ionization techniques may be used. For example, the most common ion source for analyzing elements is inductively coupled plasma (ICP). In ICP, a 10,000-degree C "flame" of plasma gas is used to atomize sample molecules and strip the outer electrons from those atoms.

Inductively coupled plasma (ICP) flame
Picture of an ICP flame viewed through green welder's glass.
The plasma is usually generated from argon gas. Plasma gas is electrically neutral overall, but a substantial number of its atoms are ionized by the high temperature.
Electron impact (EI) is another method for generating ions. In EI, the sample is heated until it becomes a gas. It is then passed through a beam of electrons. This high-energy beam strips electrons from the sample molecules, leaving behind a positively charged radical species.
Mass Analyzers
Mass analyzers separate the ions according to their mass-to-charge ratios. There are many types of mass analyzers. Each has its strengths and weaknesses, including:
- how accurately they can measure similar mass-to-charge ratios
- the range of masses and sample concentrations they can measure.
For example, a time-of-flight (TOF) analyzer uses an electric field to accelerate the ions through the same potential and then measures the time they take to reach the detector. Since the particles all have the same charge, their velocities depend only on their masses, and lighter ions will reach the detector first.
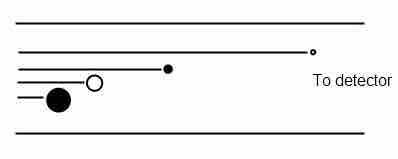
Time-of-Flight mass analyzer
Schematic of a time-of-flight (TOF) mass analyzer.
Another type of detector is a quadrupole. Here, ions are passed through four parallel rods, which apply a varying electric voltage. As the field changes, ions respond by following complex paths. Depending on the applied voltage, only ions of a certain mass-to-charge ratio will pass through the analyzer. All other ions will be lost by collision with the rods.
Using a Mass Spectrometer to Measure Mass
Here is how a mass spectrometer would analyze a sample of sodium chloride (table salt).
- In the ion source, the sample is vaporized (turned into gas) and ionized into sodium (Na+) and chloride (Cl-) ions.
- Sodium atoms and ions have only one isotope and a mass of about 23 amu.
- Chloride atoms and ions come in two isotopes, with masses of approximately 35 amu (at a natural abundance of about 75 percent) and approximately 37 amu (at a natural abundance of about 25 percent).
- The mass analyzer part of the spectrometer contains electric and magnetic fields, which exert forces on ions traveling through these fields. The angle at which the ion moves through the fields depends on its mass-to-charge ratio: lighter ions change direction more than heavier ions.
- The streams of sorted ions pass from the analyzer to the detector, which records the relative abundance of each ion type. This information is used to determine the chemical composition of the original sample (i.e. that both sodium and chlorine are present in the sample) as well as its isotopic composition (the ratio of chlorine-35 to chlorine-37).