X-ray Crystallography
X-ray crystallography is a method for determining the arrangement of atoms within a crystal structure. Substances including inorganic salts and minerals, semiconductors, and organic and biological compounds can form crystals under suitable and specific conditions. The method is useful in determining the structure of molecules, which allows researchers to characterize and understand their behavior and function. This method of structure determination has provided the most reliable evidence scientists have about the way molecules are shaped and what their bonds angles and lengths are.
The Diffraction Experiment
To obtain x-ray diffraction measurements, three components are necessary:
- a crystal sample
- a source of x-ray beams
- a detector
The best x-ray crystallographic structures are derived from the purest crystal samples, meaning samples that contain only molecules of one type and as few impurities as possible. Crystal samples contaminated with impurities, samples that are too small, and samples that are not uniform may result in the formation of imperfect crystals, whose defects affect the quality of the data that can be obtained. Once a crystal has been deemed of sufficient quality, it is generally mounted on special instruments and "shot" with an intense beam of X-rays.
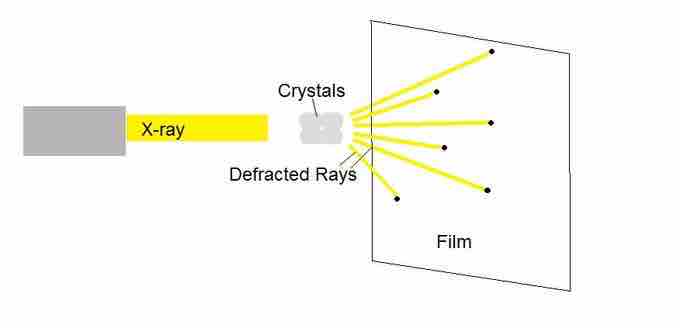
X-Ray Crystallography
When bombarded with x-ray radiation, crystals exhibit a characteristic diffraction pattern.
This process reveals the geometry of the atoms within the molecules. The x-ray beams are diffracted in a characteristic pattern that gives rise to reflections, dark spots on the detector which represent places where constructive interference of the diffracted light has occurred. The detector records the reflections on a two-dimensional surface. The crystal is typically rotated with respect to different axes and shot again with X-rays, so that diffraction patterns from all angles of the X-rays hitting the crystal are recorded.
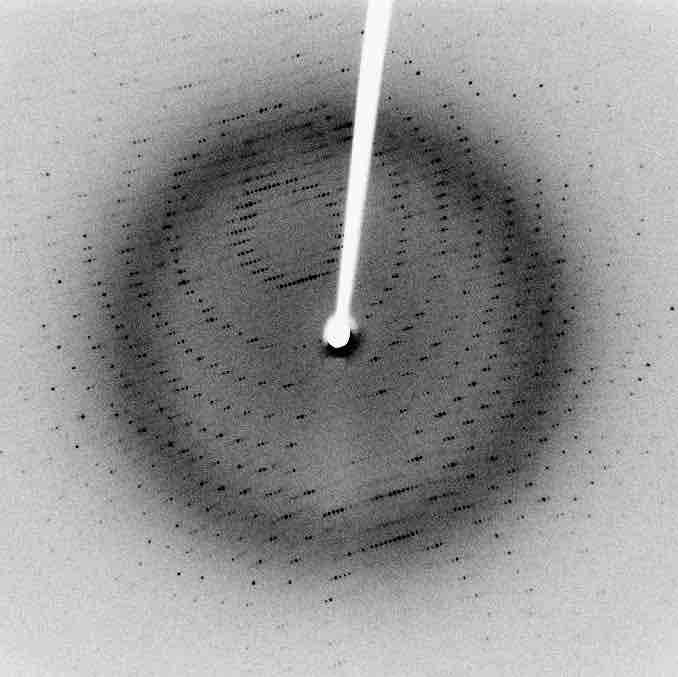
X-Ray Diffraction Pattern of a Protein
An X-ray diffraction pattern of a crystallized protein molecule. The two dimensional reflection pattern can be used to determine the atomic structure of the protein.
Then mathematical algorithms are applied in order to decode the information contained within the recorded reflections. A map is constructed to describe the electron density of the molecules in the crystal. Atomic models of the molecules are also created; these can explain the experimentally observed electron density.
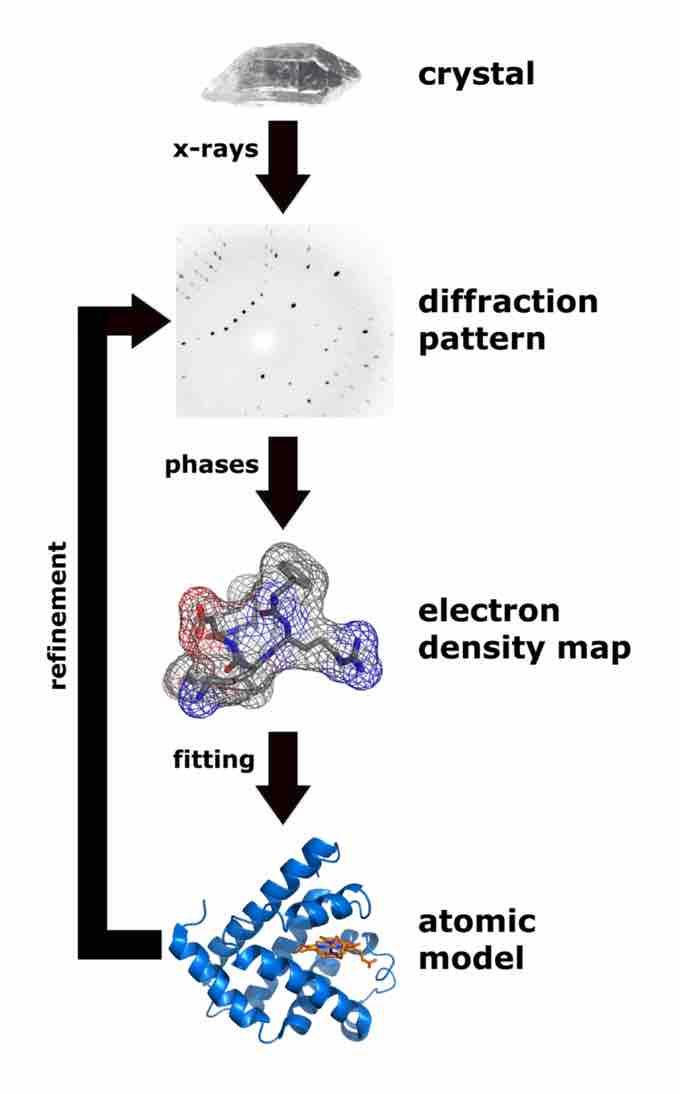
Workflow for Solving a Molecular Structure Using X-ray Crystallography
The steps to the process of determining the three dimensional structure of a molecule are outlined in this figure.
The final result is the three-dimensional structure of the molecules in the crystal. This is the most direct method that exists for "seeing" what molecules look like. Details such as atomic radii, bond angles and lengths, and molecular geometry are revealed through this method.
Historical Significance
X-ray crystallography is a powerful tool that has broad applications in the determination of the structures of both organic and inorganic compounds. Throughout the history of chemistry and biochemistry, x-ray crystallography has been one of the most important methods in helping scientists understand the atomic structure and bonding. X-ray diffraction data have proven useful in identifying the structures of protein parts of viruses, such as HIV, which was instrumental in the design of drugs that can specifically target the virus' needed machinery for its life cycle.