Concept
Version 9
Created by Boundless
The Structure and Properties of Water
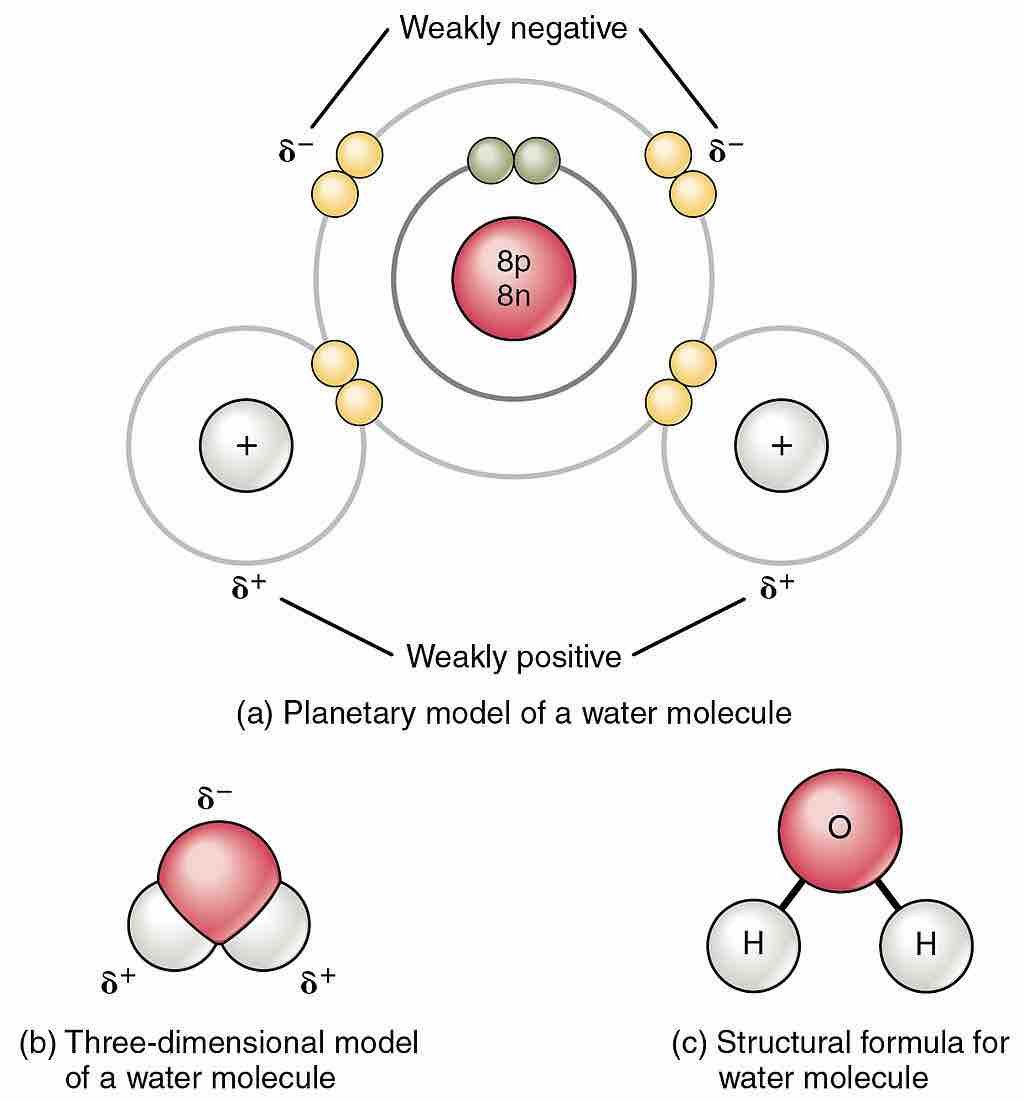
Polarity of the water molecule
Owing to the electronegativity difference between hydrogen (H) and oxygen (O) atoms, and the bent shape of the H2O molecule, a net dipole moment exists. The figure indicates the partial charges that the atoms possess.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"209 Polar Covalent Bonds in a Water Molecule."
http://commons.wikimedia.org/wiki/File:209_Polar_Covalent_Bonds_in_a_Water_Molecule.jpg
Wikimedia Commons
CC BY 3.0.