The Pauli exclusion principle, formulated by Austrian physicist Wolfgang Pauli in 1925, states that no two fermions of the same kind may simultaneously occupy the same quantum state. More technically, it states that the total wave function for two identical fermions is antisymmetric with respect to exchange of the particles. For example, no two electrons in a single atom can have the same four quantum numbers; if n, ℓ , and mℓ are the same, ms must be different such that the electrons have opposite spins.
The Pauli exclusion principle governs the behavior of all fermions (particles with half-integer spin), while bosons (particles with integer spin) are not subject to it. Fermions include elementary particles such as quarks (the constituent particles of protons and neutrons), electrons and neutrinos. In addition, protons and neutrons (subatomic particles composed from three quarks) and some atoms are fermions and are therefore also subject to the Pauli exclusion principle. Atoms can have different overall spin, which determines whether they are fermions or bosons—for example, helium-3 has spin 1/2 and is therefore a fermion, in contrast to helium-4 which has spin 0, making it a boson. As such, the Pauli exclusion principle underpins many properties of everyday matter from large-scale stability to the chemical behavior of atoms including their visibility in NMR spectroscopy.
Half-integer spin means the intrinsic angular momentum value of fermions is
The Exclusion Principle and Physical Phenomena
The Pauli exclusion principle explains a wide variety of physical phenomena. One particularly important consequence of the principle is the elaborate electron-shell structure of atoms and the way atoms share electrons. It explains the variety of chemical elements and their chemical combinations. An electrically neutral atom contains bound electrons equal in number to the protons in the nucleus. Electrons, being fermions, cannot occupy the same quantum state, so electrons have to "stack" within an atom—they have different spins while at the same place.
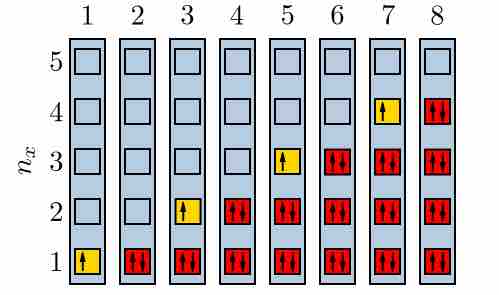
Electrons filling quantum energy levels
When a state has only one electron, it could be either spin-up or spin-down. However, according the the Pauli Exclusion Principle, when there are two in a state, there must be one of each.
An example is the neutral helium atom, which has two bound electrons, both of which can occupy the lowest-energy (1s) states by acquiring opposite spin. As spin is part of the quantum state of the electron, the two electrons are in different quantum states and do not violate the Pauli exclusion principle. However, there are only two distinct spin values for a given energy state. This property thus mandates that a lithium atom, which has three bound electrons, cannot have its third electron reside in the 1s state; it must occupy one of the higher-energy 2s states instead. Similarly, successively larger elements must have shells of successively higher energy. Because the chemical properties of an element largely depend on the number of electrons in the outermost shell, atoms with different numbers of shells but the same number of electrons in the outermost shell still behave similarly. For this reason, elements are defined by their groups and not their periods.