Volume and Density
The properties of a material may be described in many ways. Any amount of any substance will have a volume. If you have two containers of water that are different sizes, they each hold a different amount, or volume, of water. The unit for volume is a unit derived from the SI unit of length and is not a fundamental SI measurement.
If two water samples have different volumes, they still share a common measurement: the density. Density is another measurement derived from SI basic units. The density of a material is defined as its mass per unit volume. In this example, each volume of water is different and therefore has a specific and unique mass. The mass of water is expressed in grams (g) or kilograms (kg), and the volume is measured in liters (L), cubic centimeters (cm3), or milliliters (mL). Density is calculated by the dividing the mass by the volume, so that density is measured as units of mass/volume, often g/mL. If both water samples are at the same temperature, their densities should be identical, regardless of the samples' volume.
Measurement Tools

The measuring cup
The measuring cup is a common household utensil used for measuring the volumes of liquids.
If you have ever cooked in a kitchen, you have probably seen some sort of measuring cup, which allows the user to measure liquid volumes with reasonable accuracy. The measuring cup expresses liquid volume in the standard SI units of liters and milliliters. Most American measuring cups also measure liquid in the older system of cups and ounces.
Volumetric Glassware
Scientists who work in a laboratory must be familiar with typical laboratory glassware, often called volumetric glassware. These may include beakers, a volumetric flask, an Erlenmeyer flask, and a graduated cylinder. Each of these containers is used in a laboratory setting to measure liquid volumes for different purposes.

Laboratory volumetric glassware
Glassware, such as these beakers, is commonly used in a laboratory setting to conveniently measure and separate different volumes of liquids.
Density of Water
Different substances have different densities, so density is often used as a method to identify a material. Comparing the densities of two materials can also predict how substances will interact. Water is used as the common standard to substances, and it has a density of 1000 kg/m3 at Standard Temperature and Pressure (called STP).
Using Water as a Density Comparison
When an object is placed in water, the object's relative density determines whether it floats or sinks. If the object has a lower density than water, it will float to the top of the water. An object with a higher density will sink. For example, cork has a density of 240 kg/m3, so it will float. Air has a density of approximately 1.2 kg/m3, so it rises immediately to the top of a water column. The metals sodium (970 kg/m3) and potassium (860 kg/m3) will both float on water, while lead (11,340 kg/m3) will sink.
Liquids tend to form layers when added to water. The sugar alcohol glycerol (1,261 kg/m3) will sink into the water and form a separate layer until it is thoroughly mixed (glycerol is soluble in water). Vegetable oil (approx. 900 kg/m3) will float on water, and no matter how vigorously mixed, will always return as a layer on the water surface (oil is not soluble in water).
The Variable Density of Water
Water itself is a complicated and unique molecule. Even if the pressure is consistent, water's density will change based on the temperature. Recall that the three basic forms of matter are solid, liquid and gas (ignore plasma for the time being). As a rule of thumb, almost all materials are more dense in their solid or crystalline form than in their liquid form; place the solid form of almost any material on the surface of its liquid form, and it will sink. Water, on the other hand, does something very special: ice (the solid form of water) floats on liquid water.
Look carefully at the relationship between water's temperature and its density. Beginning at 100 °C, the density of water steadily increases, as far as 4 °C. At that point, the density trend reverses. At 0 °C, water freezes to ice and floats.
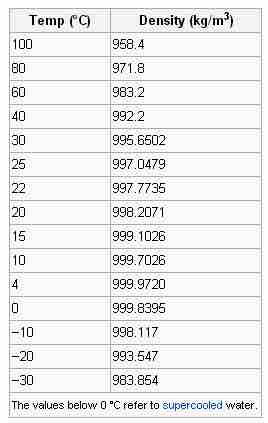
The density of water at constant pressure
This table lists the densities of water at different temperatures and constant pressure.
The implications of this simple fact are enormous: when a lake freezes, ice crusts at the surface and insulates the liquid below from freezing, while at the same time allowing the colder water (with a temp of approx. 4 °C and a high density) to sink to the bottom. If ice did not float, it would sink to the bottom, allowing more ice to form and sink, until the lake froze solid! Scuba divers and swimmers often encounter these water temperature gradients, and they might even encounter a water layer at the very bottom of a lake with a temperature of approximately 4 °C. That's just about as cold as the lake will get at the bottom; as soon as the water gets colder, the liquid water becomes less dense and rises.

Layers of water in a winter lake
During the winter months of seasonal climates, the warmest water in most lakes and rivers is only 4°C. This 4°C water has the highest density and sinks to the bottom of the lake. As the water becomes colder (<4°C), it becomes less dense and rises to form ice on the surface of the lake. As a result, liquid water always exists in lakes and rivers during the winter months. This unique property of water enables animals and plants to survive under the frozen lake or winter, ensuring that all freshwater life does not go extinct each winter.