Gas as a State of Matter
Gas is one of the three classical states of matter (the others being liquid and solid). Near absolute zero, a substance exists as a solid. As heat is added to this substance it melts into a liquid at its melting point, boils into a gas at its boiling point, and if heated high enough would enter a plasma state in which the electrons are so energized that they leave their parent atoms from within the gas.

States of matter
Matter transitions between three classical states of matter (gas, solid, and liquid) and a fourth state of matter. Note that the enthalpy of a system is the heat content of a system at constant pressure. The process of a solid converting to a liquid is known as "melting"; liquid to a gas is "vaporization"; and gas back to a solid is "deposition." These same processes in the reverse direction are "freezing"; "condensation"; and "sublimation."
Kinetic Theory of Matter
Why does matter exist in three different states? The Kinetic Theory of Matter provides a basic overview:
- Matter is made up of constantly-moving particles.
- All particles have energy, but the energy varies depending on whether the substance is a solid, liquid, or gas; solid particles have the least amount of energy and gas particles the most.
- The temperature of a substance is a measure of the average kinetic energy of the particles.
- A change in phase may occur when the energy of the particles changes.
- There are spaces between the particles of matter.
- There are attractive forces between particles and these become stronger as the particles move closer together. These attractive forces are known as intermolecular forces. An ideal gas is assumed to experience no intermolecular forces whatsoever, due to the fact that the particles of an ideal gas are moving so quickly, and are so far apart from one another, that they do not interact at all.
- Gases behave most ideally at high temperatures and low pressures. This is because under these conditions, intermolecular forces will be minimized.
We discussed the basic states of matter in which a substance can be interconverted depending on conditions. Under standard conditions (1 atm, 273 K), a substance which exists as a gas is called a pure gas and (disregarding any substance-specific intermolecular forces or particle volume that could alter this value) has a volume of 22.4 L per mole. A pure gas may be made up of individual atoms (e.g. a noble gas or atomic gas like neon), elemental molecules made from one type of atom (e.g. oxygen), or compound molecules made from a variety of atoms (e.g. carbon dioxide). At STP, if the boiling point of a given substance is below 273 K then you would expect that substance to be in gas form.
Elemental Gases
The group VIII elements (helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe) and radon (Rn)) exist as monatomic gases at standard temperature and pressure (STP) and are called the noble gases. The only other elements which exist as gases at STP are hydrogen (H2), nitrogen (N2) and oxygen (O2), plus the two halogens, fluorine (F2) and chlorine (Cl2). These gases, when grouped together with the monatomic noble gases are called "elemental gases. "
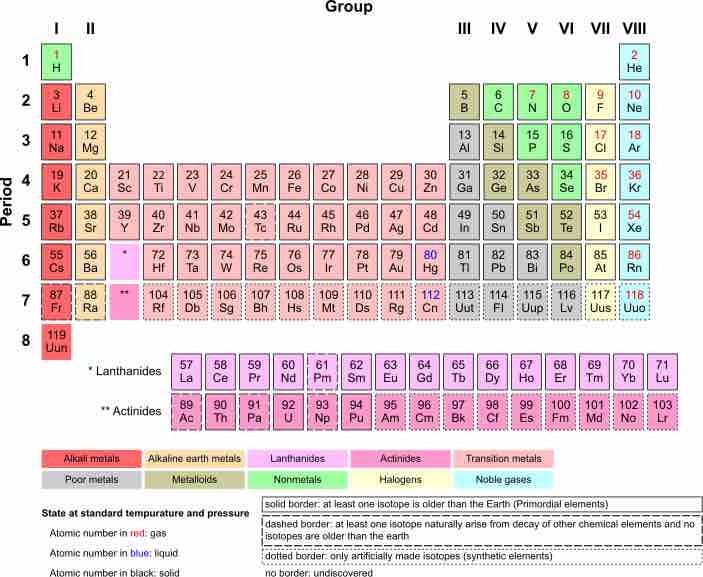
Periodic Table of Elements
Nobles Gases (Group VIII) exist as gases at STP.