Concept
Version 13
Created by Boundless
Collecting Gases Over Water
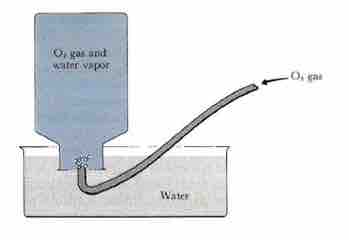
Collecting gas over water
When collecting oxygen gas and calculating its partial pressure by displacing water from an inverted bottle, the presence of water vapor in the collecting bottle must be accounted for; this is easily accomplished using Dalton's Law of Partial Pressures.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Chemical Principles/Gas Laws and the Kinetic Theory."
http://en.wikibooks.org/wiki/Chemical_Principles/Gas_Laws_and_the_Kinetic_Theory
Wikibooks
CC BY-SA 3.0.