Concept
Version 13
Created by Boundless
Distribution of Molecular Speeds and Collision Frequency
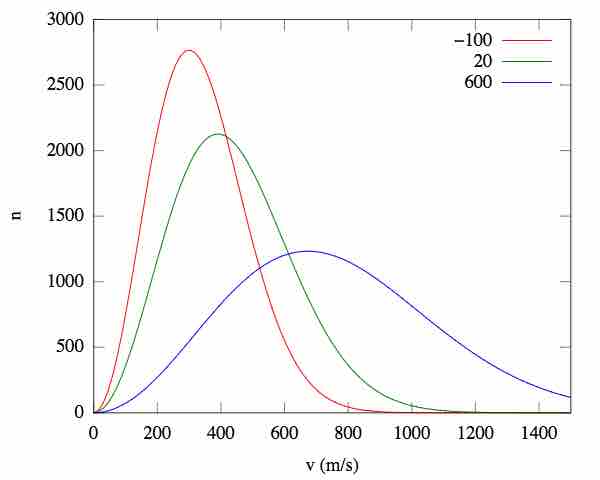
Effect of temperature on root-mean-square speed distributions
As the temperature increases, so does the average kinetic energy (v), resulting in a wider distribution of possible velocities. n = the fraction of molecules.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Maxwell-Boltzmann_distribution.svg."
https://commons.wikimedia.org/wiki/File:Maxwell-Boltzmann_distribution.svg
Wikimedia
CC BY-SA 3.0.