A reaction is said to be second-order when the overall order is two. For a reaction with the general form
or
The second scenario, in which the reaction is first-order in both A and B, would yield the following rate law:
Applying the Method of Initial Rates to Second-Order Reactions
Consider the following set of data:
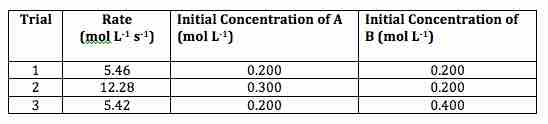
Rates and initial concentrations for A and B
A table showing data for three trials measuring the various rates of reaction as the initial concentrations of A and B are changed.
If we are interested in determining the order of the reaction with respect to A and B, we apply the method of initial rates.
Determining Reaction Order in A
In order to determine the reaction order for A, we can set up our first equation as follows:
Note that on the right side of the equation, both the rate constant k and the term
Therefore, the reaction is second-order in A.
Determining Reaction Order in B
Next, we need to determine the reaction order for B. We do this by picking two trials in which the concentration of B changes, but the concentration of A does not. Trials 1 and 3 will do this for us, and we set up our ratios as follows:
Note that both k and the concentrations of A cancel. Also,
Therefore, the reaction is zero-order in B.
Overall Reaction Order
We have determined that the reaction is second-order in A, and zero-order in B. Therefore, the overall order for the reaction is second-order