Concept
Version 15
Created by Boundless
Representing Valence Electrons in Lewis Symbols
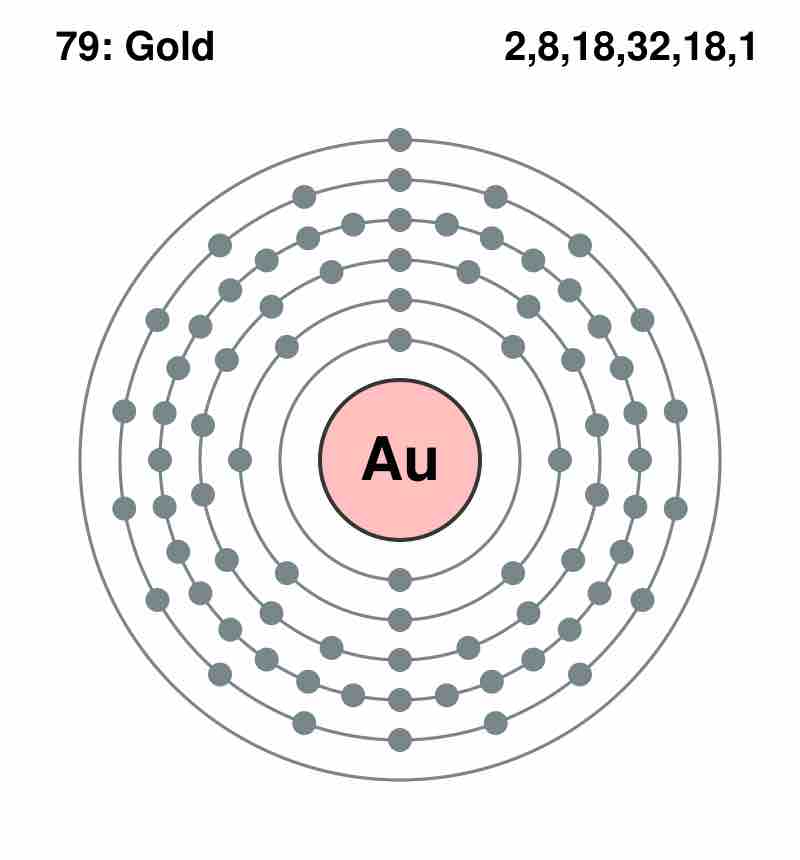
Principal energy levels of gold (Au)
The figure shows the organization of the electrons around the nucleus of a gold (Au) atom. Notice that the first energy level (closest to the nucleus) can have only two electrons, while more electrons can 'fit' within a given level further out. The number of electrons in each level is listed on the upper right corner of the figure. Notice that the outermost level has only one electron.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Electron shell diagram for Gold."
http://commons.wikimedia.org/wiki/File:Electron_shell_079_Gold.svg
Wikimedia
CC BY 2.0 UK: England & Wales.