Concept
Version 8
Created by Boundless
Bond Lengths
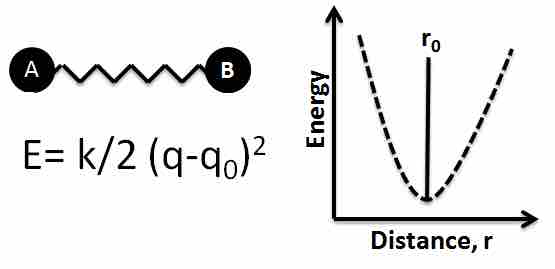
Ball-and-spring model of a chemical bond
A bond between two atoms can be thought of as a spring with two balls attached to it. Any stretch or compression of the spring will initiate oscillations of the atoms with respect to their equilibrium (unperturbed) positions. The potential energy function for this system is also indicated. The minimum energy occurs at the equilibrium distance r0, which is where the bond length is measured.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Ball and Spring Model and Corresponding Potential Energy Diagram."
https://commons.wikimedia.org/wiki/File:Ball_and_Spring_Model_and_Corresponding_Potential_Energy_Diagram.png
Wikimedia Commons
CC BY-SA 3.0.