Early History of the Atom
Matter is composed of indivisible building blocks. This idea was recorded as early as the fifth century BCE by Leucippus and Democritus. The Greeks called these particles atomos, meaning indivisible, and the modern word "atom" is derived from this term. Democritus proposed that different types and combinations of these particles were responsible for the various forms of matter. However, these ideas were largely ignored at the time, as most philosophers favored the Aristotelian perspective.
The concept of the atom was revisited and elaborated upon by many scientists and philosophers, including Galileo, Newton, Boyle, and Lavoisier. In 1661, Boyle presented a discussion of atoms in his The Sceptical Chymist. However, the English chemist and meteorologist John Dalton is credited with the first modern atomic theory, as explained in his A New System of Chemical Philosophy.
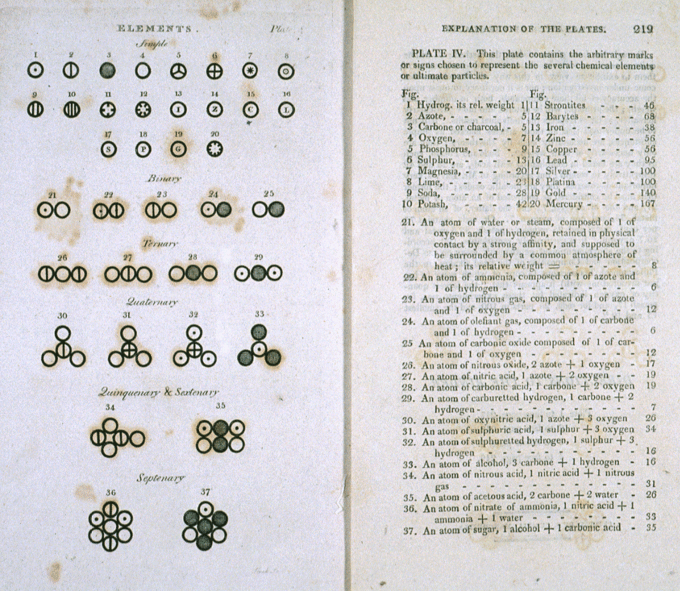
John Dalton's A New System of Chemical Philosophy
Chemical structures from Dalton's A New System of Chemical Philosophy.
Dalton's experiments with gases led to some of the earliest measurements of atomic masses and a concept of atomic structure and reactivity. Dalton's atomic theory contained the following ideas:
- All atoms of a given element are identical.
- The atoms of different elements vary in mass and size.
- Atoms are indestructible. Chemical reactions may result in their rearrangement, but not their creation or destruction.
Dalton also outlined a law of multiple proportions, which described how reactants will combine in set ratios. Like the early philosophers, Dalton's theories were not popularly accepted for much of the 19th century, but his ideas have since been accepted, with amendments addressing subatomic particles and the interconversion of energy and mass.