Molecular formulas describe the exact number and type of atoms in a single molecule of a compound. The constituent elements are represented by their chemical symbols, and the number of atoms of each element present in each molecule is shown as a subscript following that element's symbol. The molecular formula expresses information about the proportions of atoms that constitute a particular chemical compound, using a single line of chemical element symbols and numbers. Sometimes it also includes other symbols, such as parentheses, dashes, brackets, and plus (+) and minus (–) signs.
For organic compounds, carbon and hydrogen are listed as the first elements in the molecular formula, and they are followed by the remaining elements in alphabetical order. For example, for butane, the molecular formula is C4H10. For ionic compounds, the cation precedes the anion in the molecular formula. For example, the molecular formula of sodium fluoride is NaF.
A molecular formula is not a chemical name, and it contains no words. Although a molecular formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Molecular formulas are more limiting than chemical names and structural formulas.
Empirical and Molecular Formulas
The simplest types of chemical formulas are called empirical formulas, which indicate the ratio of each element in the molecule. The empirical formula is the simplest whole number ratio of all the atoms in a molecule. For example:
- The molecular formula for glucose is C6H12O6. The molecular formula indicates the exact number of atoms in the molecule.
- The empirical formula expresses the smallest whole number ratio of the atoms in the element. In this case, the empirical formula of glucose is CH2O.
To convert between empirical and molecular formulas, the empirical formula can be multiplied by a whole number to reach the molecular formula. In this case, the empirical formula would be multiplied by 6 to get to the molecular formula.
Examples of Empirical and Molecular Formulas:
- The compound dichlorine hexoxide has an empirical formula ClO3 and the molecular formula Cl2O6
- The compound hydrogen peroxide has the empirical formula HO and the molecular formula H2O2
Molecular Formulas and Structural Formulas
Molecular formulas contain no information about the arrangement of atoms. Because of this, one molecular formula can describe a number of different chemical structures. A structural formula is used to indicate not only the number of atoms, but also their arrangement in space. A structural formula is not as compact and easy to communicate, but it provides information that the molecular formula does not about the relative positioning of atoms and the bonding between atoms. Compounds that share a chemical formula but have different chemical structures are known as isomers, and they can have quite different physical properties.
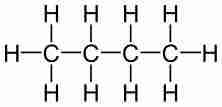
Structural formula of butane
The chemical structure of butane indicates not only the number of atoms, but also their arrangement in space.