Ionic Bonds in Reality
When two elements form an ionic compound, is an electron really lost by one atom and transferred to the other? To answer this question, consider the data on the ionic solid LiF. The average radius of the neutral Li atom is about 2.52Å. If this Li atom reacts with an F atom to form LiF, what is the average distance between the Li nucleus and the electron it has "lost" to the fluorine atom? The answer is 1.56Å; the electron is now closer to the lithium nucleus than it was in neutral lithium.
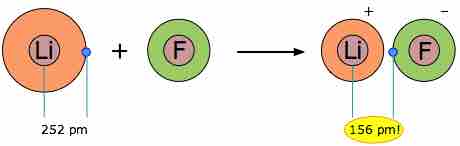
Bonding in lithium fluoride
Where is the electron in lithium fluoride? Does this make an ionic bond, a covalent bond, or something in between?
The answer to the above question is both yes and no: yes, the electron that was now in the 2s orbital of Li is now within the grasp of a fluorine 2p orbital; but no, the electron is now even closer to the Li nucleus than before, so it is not truly "lost."
The electron-pair bond is clearly responsible for this situation; this provides the covalent bond's stability. What is not as obvious—until you look at the numbers such as are quoted for LiF above—is that the ionic bond results in the same condition; even in the most highly ionic compounds, both electrons are close to both nuclei, and the resulting mutual attractions bind the nuclei together.
The emerging view of ionic bonding is one in which the electron orbitals of adjacent atom pairs are simply skewed, placing more electron density around the "negative" element than around the "positive" one. Think of this skewing's magnitude as the percent ionic character of a bond; to determine the percent ionic character, one must look at the electronegativities of the atoms involved and determine how effective the electron sharing is between the species.
The ionic bonding model is useful for many purposes, however. There is nothing wrong with using the term "ionic bond" to describe the interactions between the atoms in the very small class of "ionic solids" such as LiF and NaCl.
Bond Angle
A bond angle forms between three atoms across at least two bonds. The more covalent in nature the bond, the more likely the atoms will situate themselves along the predetermined vectors given by the orbitals that are involved in bonding (VSEPR theory). The more ionic character there is to a bond, the more likely that non-directional electrostatic interactions are holding the atoms together. This means that atoms will sit in positions that minimize the amount of space they occupy (like a salt crystal).