A base is a substance that can accept hydrogen ions (H+) or, more generally, donate a pair of valence electrons. A weak base is a chemical base that does not ionize fully in an aqueous solution. As Brønsted-Lowry bases are proton acceptors, a weak base may also be defined as a chemical base with incomplete protonation. A general formula for base behavior is as follows:
A base can either accept protons from water molecules or donate hydroxide ions to a solution. Both actions raise the pH of the solution by decreasing the concentration of H+ ions. This results in a relatively low pH compared to that of strong bases. The pH of bases in aqueous solution ranges from greater than 7 (the pH of pure water) to 14 (though some bases have pH values greater than 14). The formula for pH is:
pH = -log10[H+]
Sometimes, however, it is more convenient to focus on the pOH of bases, rather than the pH. The pOH more directly references the [OH-].
pOH = -log10[OH-]
Some common weak bases and their corresponding pKb values include:
- C6H5NH2 (9.38)
- NH3 (4.75)
- CH3NH2 (3.36)
- CH3CH2NH2 (3.27)
Smaller pKb values indicate higher values of Kb; this also indicates a stronger base.
Like weak acids, weak bases have important applications in biochemical studies, chemistry reactions, and physiological purposes, particularly because of their role in buffer solutions. Weak bases can also be used to catalyze certain reactions, such as enolate formation, as demonstrated in the figure below:
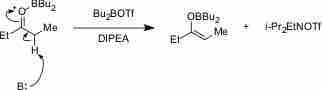
Weak base catalyzing enolate formation
A weak base, symbolized by B:, can catalyze enolate formation by acting as a proton acceptor.