Section 2
Strength of Acids
Book
Version 33
By Boundless
By Boundless
Boundless Chemistry
Chemistry
by Boundless
3 concepts
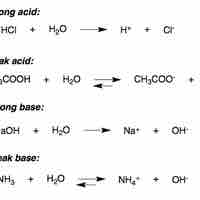
Strong Acids
In water, strong acids completely dissociate into free protons and their conjugate base.

Weak Acids
A weak acid only partially dissociates in solution.
Calculating Percent Dissociation
Percent dissociation represents an acid's strength and can be calculated using the Ka value and the solution's pH.