As their name suggests, polyprotic acids contain more than one acidic proton. Two common examples are carbonic acid (H2CO3, which has two acidic protons and is therefore a diprotic acid) and phosphoric acid (H3PO4, which has three acidic protons and is therefore a triprotic acid).
Diprotic and polyprotic acids show unique profiles in titration experiments, where a pH versus titrant volume curve clearly shows two equivalence points for the acid; this is because the two ionizing hydrogens do not dissociate from the acid at the same time. With any polyprotic acid, the first amd most strongly acidic proton dissociates completely before the second-most acidic proton even begins to dissociate.
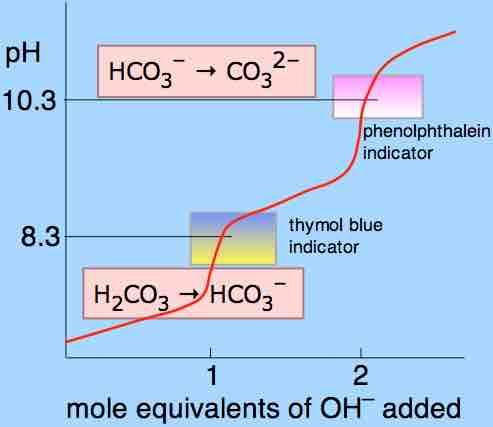
Titration curve of carbonic acid
The titration curve of a polyprotic acid has multiple equivalence points, one for each proton. In carbonic acid's case, the two ionizing protons each have a unique equivalence point.
Diprotic Acids
A diprotic acid (here symbolized by H2A) can undergo one or two dissociations depending on the pH. Dissociation does not happen all at once; each dissociation step has its own Ka value, designated Ka1 and Ka2:
The first dissociation constant is necessarily greater than the second ( i.e. Ka1 > Ka2); this is because the first proton to dissociate is always the most strongly acidic, followed in order by the next most strongly acidic proton. For example, sulfuric acid (H2SO4) can donate two protons in solution:
This first dissociation step of sulfuric acid will occur completely, which is why sulfuric acid is considered a strong acid; the second dissociation step is only weakly dissociating, however.
Triprotic Acids
A triprotic acid (H3A) can undergo three dissociations and will therefore have three dissociation constants: Ka1 > Ka2 > Ka3. Take, for example the three dissociation steps of the common triprotic acid phosphoric acid:
Fractional Concentration of Conjugate Base Species
Although the subsequent loss of each hydrogen ion is less favorable, all of a polyprotic acid's conjugate bases are present to some extent in solution. Each species' relative level is dependent on the pH of the solution. Given the pH and the values of Ka for each dissociation step, we can calculate each species' fractional concentration, α (alpha). The fractional concentration is defined as the concentration of a particular conjugate base of interest, divided by the sum of all species' concentrations. For example, a generic diprotic acid will generate three species in solution: H2A, HA-, and A2-, and the fractional concentration of HA-, which is given by:
The following formula shows how to find this fractional concentration of HA-, in which pH and the acid dissociation constants for each dissociation step are known:
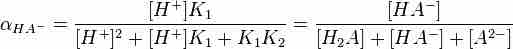
Fractional ion calculations for polyprotic acids
The above complex equations can determine the fractional concentration of various ions from polyprotic acids.