Concept
Version 9
Created by Boundless
Strong Acid-Weak Base Titrations
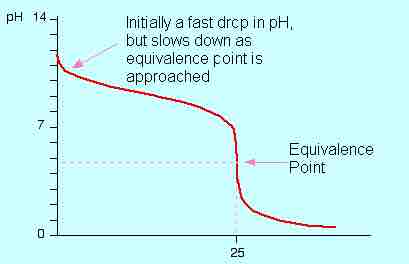
Titration of a weak base with a strong acid
A depiction of the pH change during a titration of HCl solution into an ammonia solution. The curve depicts the change in pH (on the y-axis) vs. the volume of HCl added in mL (on the x-axis).
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Boundless."
http://s3.amazonaws.com/figures.boundless.com/50a168a0e4b04ac1150c0c72/tit1.png
Amazon Web Services
License: Other.