Concept
Version 9
Created by Boundless
Hydrocarbons
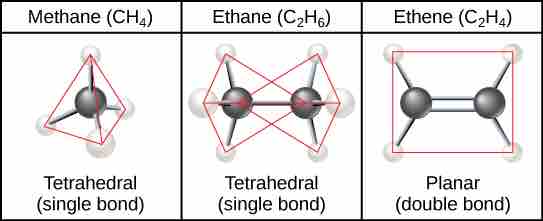
Hydrocarbon Chains
When carbon forms single bonds with other atoms, the shape is tetrahedral. When two carbon atoms form a double bond, the shape is planar, or flat. Single bonds, like those found in ethane, are able to rotate. Double bonds, like those found in ethene cannot rotate, so the atoms on either side are locked in place.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"OpenStax College, Carbon. October 16, 2013."
http://cnx.org/content/m44393/latest/Figure_02_03_02.jpg
OpenStax CNX
CC BY 3.0.