Tonicity in Living Systems
Tonicity is the ability of a solution to exert an osmotic pressure upon a membrane. There are three types of tonicity: hypotonic, hypertonic, and isotonic. In a hypotonic environment, water enters a cell, and the cell swells. In a hypertonic solution, water leaves a cell and the cell shrinks. In an isotonic condition, the relative concentrations of solute and solvent are equal on both sides of the membrane. There is no net water movement; therefore, there is no change in the size of the cell. If either the hypo- or hyper- condition goes to excess, the cell's functions become compromised, and the cell may be destroyed.
A red blood cell will burst, or lyse, when it swells beyond the plasma membrane's capability to expand. The membrane resembles a mosaic with discrete spaces between the molecules comprising it. If the cell swells and the spaces between the lipids and proteins become too large, the cell will break apart.
In contrast, when excessive amounts of water leave a red blood cell, the cell shrinks, or crenates. This has the effect of concentrating the solutes left in the cell, making the cytosol denser and interfering with diffusion within the cell. The cell's ability to function will be compromised and may also result in the death of the cell.
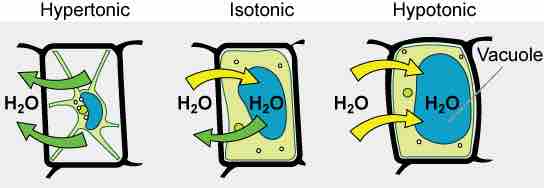
Turgor Pressure and Tonicity in a Plant Cell
The turgor pressure within a plant cell depends on the tonicity of the solution in which it is bathed.
Osmoregulation
Various living things have ways of controlling the effects of osmosis—a mechanism called osmoregulation. Some organisms, such as plants, fungi, bacteria, and some protists, have cell walls that surround the plasma membrane and prevent cell lysis in a hypotonic solution. The plasma membrane can only expand to the limit of the cell wall, so the cell will not lyse. In fact, in plants, the cellular environment is always slightly hypotonic to the cytoplasm, and water will always enter a cell if water is available. This inflow of water produces turgor pressure, which stiffens the cell walls of the plant. In nonwoody plants, turgor pressure supports the plant. Conversely, if the plant is not watered, the extracellular fluid will become hypertonic, causing water to leave the cell . In this condition, the cell does not shrink because the cell wall is not flexible. However, the cell membrane detaches from the wall and constricts the cytoplasm. This is called plasmolysis. Plants lose turgor pressure in this condition and wilt .

Turgor Pressure and Plasmolysis
Without adequate water, the plant on the left has lost turgor pressure, visible in its wilting; the turgor pressure is restored by watering it (right).
Tonicity is a concern for all living things. For example, paramecia and amoebas, which are protists that lack cell walls, have contractile vacuoles. This vesicle collects excess water from the cell and pumps it out, keeping the cell from lysing as it takes on water from its environment .
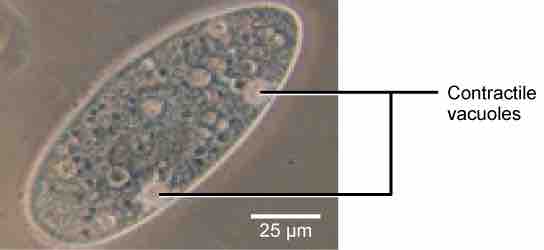
Contractile Vacuoles
A paramecium's contractile vacuole, here visualized using bright field light microscopy at 480x magnification, continuously pumps water out of the organism's body to keep it from bursting in a hypotonic medium.
Many marine invertebrates have internal salt levels matched to their environments, making them isotonic with the water in which they live. Fish, however, must spend approximately five percent of their metabolic energy maintaining osmotic homeostasis. Freshwater fish live in an environment that is hypotonic to their cells. These fish actively take in salt through their gills and excrete diluted urine to rid themselves of excess water. Saltwater fish live in the reverse environment, which is hypertonic to their cells, and they secrete salt through their gills and excrete highly concentrated urine.
In vertebrates, the kidneys regulate the amount of water in the body. Osmoreceptors are specialized cells in the brain that monitor the concentration of solutes in the blood. If the levels of solutes increase beyond a certain range, a hormone is released that retards water loss through the kidney and dilutes the blood to safer levels. Animals also have high concentrations of albumin (produced by the liver) in their blood. This protein is too large to pass easily through plasma membranes and is a major factor in controlling the osmotic pressures applied to tissues.