Predicting Disease Risk at the Individual Level
The introduction of DNA sequencing and whole genome sequencing projects, particularly the Human Genome project, has expanded the applicability of DNA sequence information. Genomics is now being used in a wide variety of fields, such as metagenomics, pharmacogenomics, and mitochondrial genomics. The most commonly-known application of genomics is to understand and find cures for diseases.
Predicting the risk of disease involves screening currently-healthy individuals by genome analysis at the individual level. Intervention with lifestyle changes and drugs can be recommended before disease onset. However, this approach is most applicable when the problem resides within a single gene defect. Such defects only account for approximately five percent of diseases in developed countries. Most of the common diseases, such as heart disease, are multi-factored or polygenic, which is a phenotypic characteristic that involves two or more genes interacting with environmental factors such as diet. In April 2010, scientists at Stanford University published the genome analysis of a healthy individual (Stephen Quake, a scientist at Stanford University, who had his genome sequenced); the analysis predicted his propensity to acquire various diseases. A risk assessment was performed to analyze Quake's percentage of risk for 55 different medical conditions. A rare genetic mutation was found, which showed him to be at risk for sudden heart attack. He was also predicted to have a 23 percent risk of developing prostate cancer and a 1.4 percent risk of developing Alzheimer's. The scientists used databases and several publications to analyze the genomic data. Even though genomic sequencing is becoming more affordable and analytical tools are becoming more reliable, ethical issues surrounding genomic analysis at a population level remain to be addressed.
In 2011, the United States Preventative Services Task Force recommended against using the PSA test to screen healthy men for prostate cancer. Their recommendation was based on evidence that screening does not reduce the risk of death from prostate cancer. Prostate cancer often develops very slowly and does not cause problems, while the cancer treatment can have severe side effects. The PCA3 test is considered to be more accurate, but screening may still result in men suffering side effects from treatment who would not have been harmed by the cancer itself .
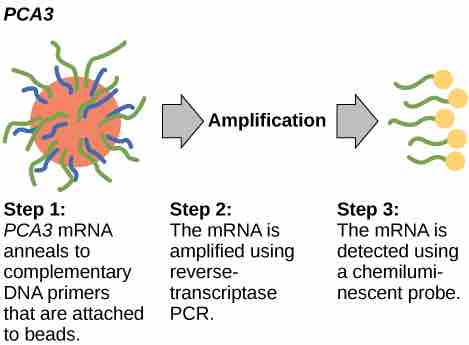
PCA3
PCA3 is a gene that is expressed in prostate epithelial cells and overexpressed in cancerous cells. A high concentration of PCA3 in urine is indicative of prostate cancer.